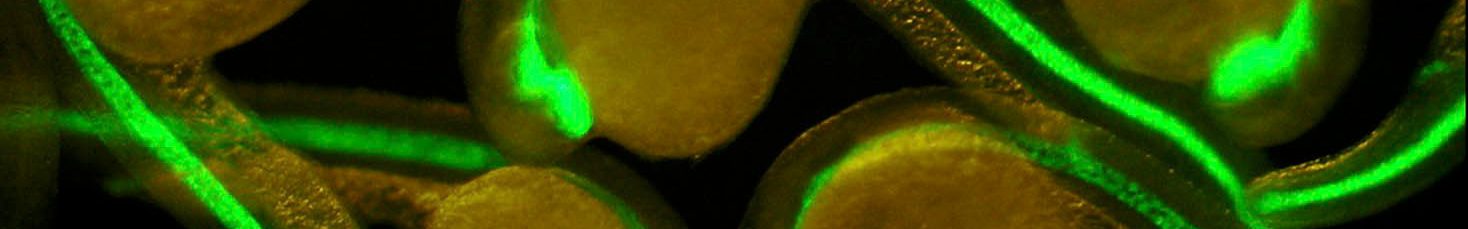
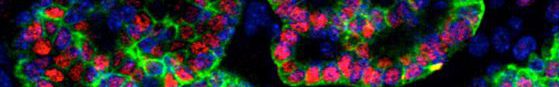
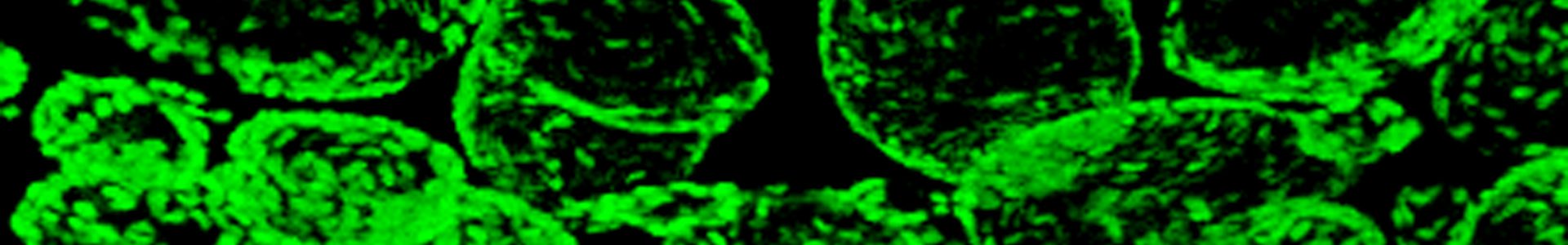
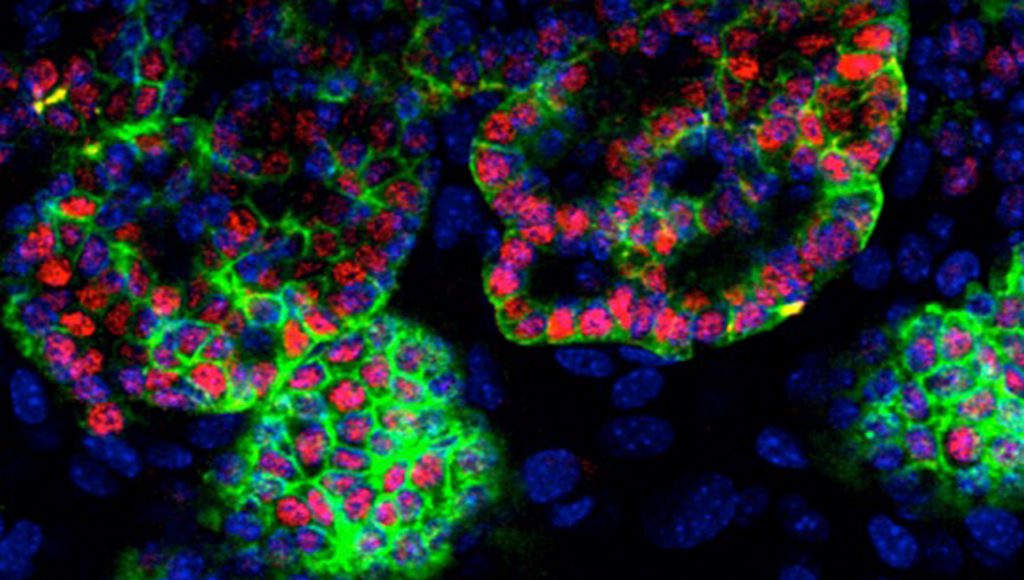
- ES cells derived thyroid follicles
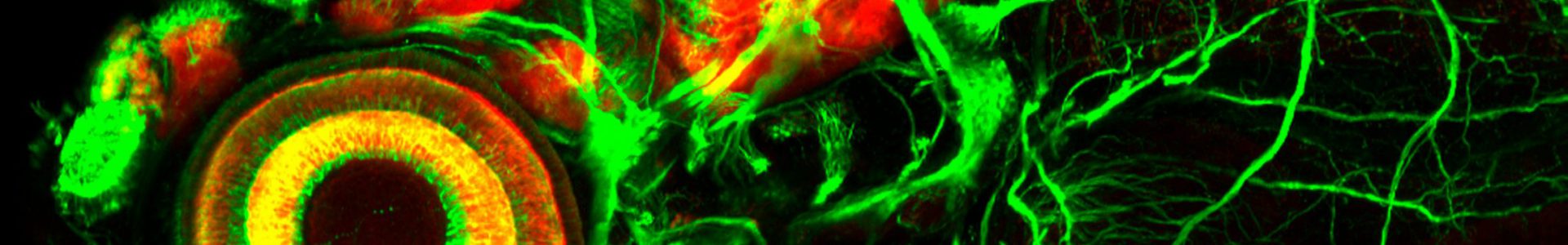
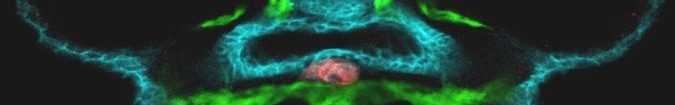
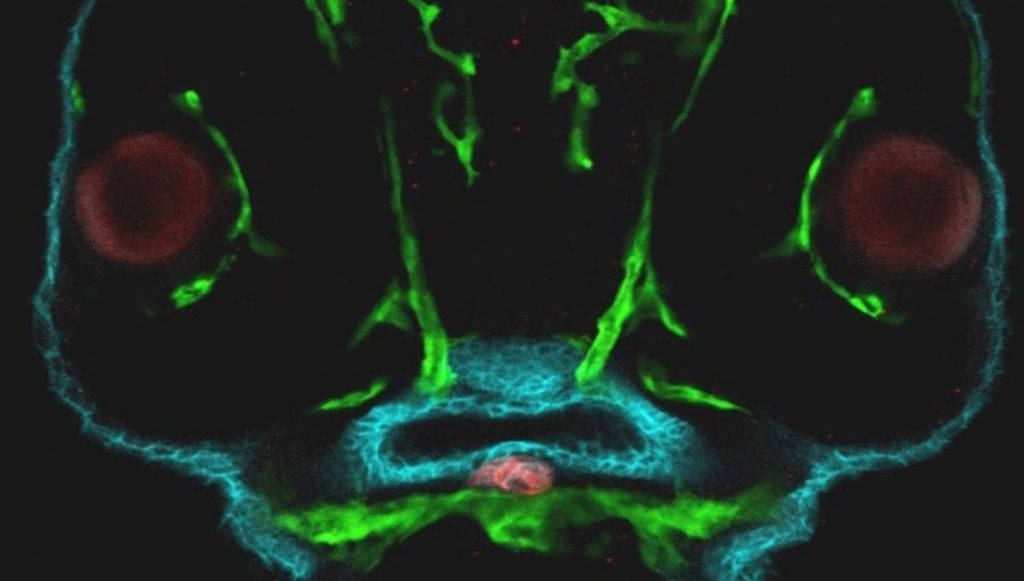
- Zebrafish embryo. Thyroid (Red) – Endoderm (Blue) – Vasculature (green)
The main research focus of our team is the molecular dissection of the signaling mechanisms and gene networks involved in thyroid organogenesis and thyroid diseases (congenital hupothyroidism and thyroid cancer). To date, the signaling mechanisms that control the specification of endoderm-derived organs such as the thyroid, lung, liver or pancreas remain poorly understood. To tackle the question « how different cell types are specified from the gut endoderm? », we use the thyroid as experimental model. Thyroid organogenesis can be grossly divided into 3 phases comprising (a) thyroid precursor cell specification, (b) budding and migration of the thyroid primordium, and (c) functional differentiation of thyroid follicular cells. While some factors critical for migration, differentiation and growth of the thyroid primordium have been identified in mouse knock-out models, little is known about the molecular events involved in thyroid precursors cell specification.
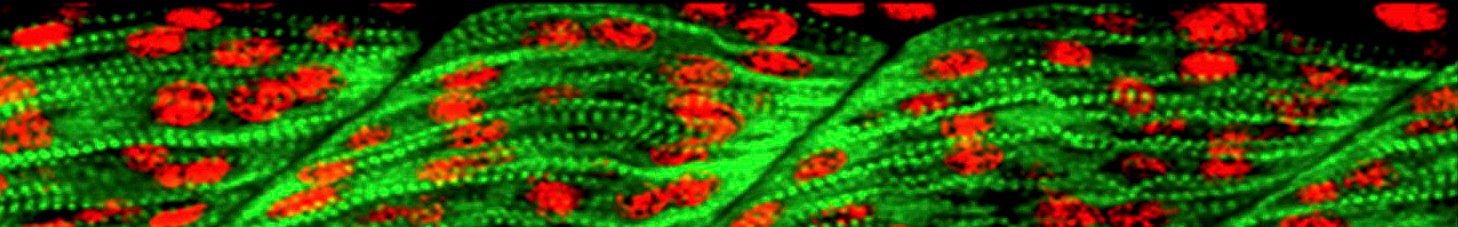
Our working strategy aims to merge data obtained with two different experimental models – Thyroid cells differentiated in vitro from genetically modified ES cells, from IPSc and zebrafish – to elucidate the genetic basis of thyroid development and the molecular basis of thyroid cell differentiation.
Based on data obtained from global expression analysis of normal thyroid development In vitro or in zebrafish as well as from models of thyroid dysgenesis, a reverse genetic approach is applied by using CRISPR/Cas for creating knockout alleles of candidate genes in ES cells and zebrafish.
![]()

This will help:
-to offer new diagnostic targets (=new candidates genes) to patients with thyroid dysgenesis.
-to identify (i) the developmental thyroid tempo orchestrated by some signaling pathways controlled by the cardio vascular system which (ii) would help to build in vitro models where thyroid differentiation could be trigger by chemically defined culture conditions and in absence of any genetic manipulation.
This last achievement would pave the way for a human model of in vitro thyroid differentiation and could offer unprecedented therapeutic options to patients with thyroid dysgenesis or medical thyroid ablation.