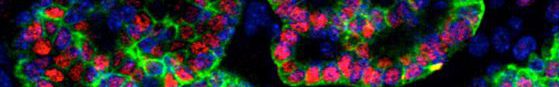
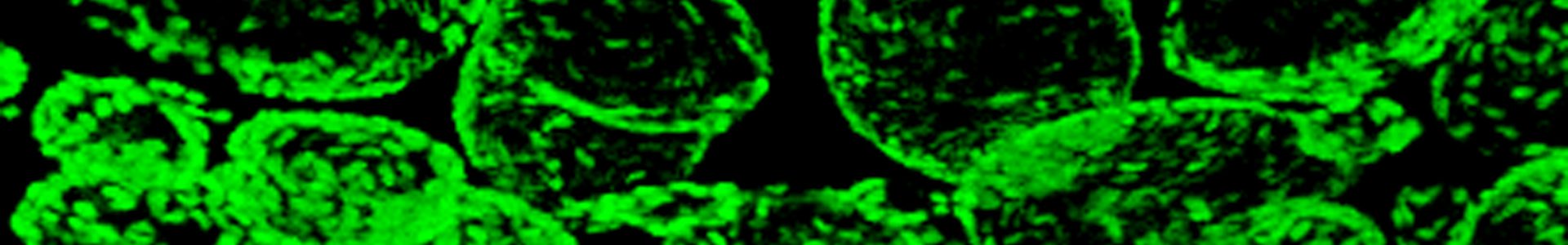
The main goal of the present project is to use pluripotent stem cells to generate in vitro models of thyroid cancer.
Identifying the early events that contribute to cancer initiation and/or progression would have major implications in thyroid cancer diagnosis and therapy. In the thyroid field, in absence of a genetically tractable model, our knowledge of molecular initiating events of thyroid cancer remains limited. Importantly, no publications have been reported so far using an in vitro ES cells-derived model for thyroid cancer initiation.
Our lab has demonstrated the generation of functional thyroid tissue in a three-dimensional mouse embryonic stem cell culture system. In this work, we first report how doxycycline induction of two transcription factors, NKX2.1 and PAX8, directs mESC differentiation towards a thyroid follicular cell (TFC) fate. Then, we demonstrate that subsequent treatment with recombinant human TSH promotes efficient self-assembly of TFC-like cells into three-dimensional organoids displaying molecular, morphological and functional properties of bona fide thyroid follicles. Finally, we clearly demonstrate that mESC-derived TFC, when grafted under the kidney capsules of female hypothyroid mice, develop into functional thyroid tissue capable of rescuing thyroid hormone deficits.
In our cancer project, we will use cells genetically engineered to conditionally express (tamoxifen-inducible system) the thyroid oncogenes BRAFV637E (Papillary thyroid carcinoma model), RASQ61R (Follicular thyroid carcinoma model), or the combination of BRAFV637E and p53 knockdown (Anaplastic thyroid carcinoma model) to create different thyroid cancer in vitro models. We will use these models to investigate: (a) the effect of thyroid oncogenes induction at different stages of thyroid development; (b) early events involved in thyroid cancer initiation; (c) the effect of inactivation of p53 on thyroid cancer progression; (d) mechanisms involved in drug resistance