PhD students
2 positions are open in 2024 ! Please apply via our International PhD programme → https://iribhmphd.ulb.be/
Position in organoids research for organ specification
The Costagliola lab, at IRIBHM, ULB, Brussels, (https://costalab.ulb.ac.be/ ; https://iribhm.org/) is seeking a creative and highly motivated PhD student. This project aims to use organoid technology to explore the role of cardiac mesoderm on the thyroid specification process from foregut endoderm.
Project Background
Thyroid tissue, the site of de novo thyroid hormone biosynthesis, is derived from ventral pharyngeal endoderm and defects in morphogenesis are a predominant cause of congenital hypothyroidism (1/3000). It is remarkable that there is an increased prevalence of cardiovascular malformations in people with defects in thyroid morphogenesis. The first molecularly recognizable step of thyroid development is the specification of thyroid precursors in anterior foregut endoderm. Recent studies have identified crucial roles of FGF and BMP signaling in thyroid specification, but the interplay between signaling cues and thyroid transcription factors remained elusive. Observations made in different species consistently point to precardiac and cardiac mesoderm as a major source of FGF and BMP ligands acting on the foregut endoderm to initiate thyroid cell differentiation. In all species analyzed, the thyroid anlage is specified in the region of the foregut endoderm that is immediately adjacent to the cardiac mesoderm forming the outflow tract of the developing heart. Zebrafish studies also showed that loss of cardiac mesoderm, impaired myocardial differentiation, or perturbed positioning of cardiac mesoderm relative to the prospective thyroid field in the foregut endoderm results in aberrant thyroid development.
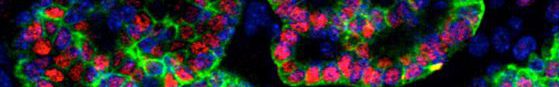
Our laboratory excels in the generation of thyroid organoids from both murine and human pluripotent stem cells. The objective of this study is to utilize this organoid technology to investigate the dynamic expression patterns of NKX2.1 and Pax8, the key transcription factors implicated in thyroid specification and development. Employing a strategy involving co-culturing of in vitro derived cardiac mesodermal cells with in vitro derived foregut endoderm, we aim to delineate the influence of cardiac mesoderm on promoting the emergence of a progenitor population from the foregut endoderm, subsequently differentiating into lineage-committed thyroid precursors co-expressing NKX2.1 and Pax8. At various time point, single-cell RNA sequencing (scRNAseq) and single-cell assay for transposase-accessible chromatin sequencing (scATACseq) will be performed to decipher the molecular and genomic events underlying this precisely timed process. Additionally, these analyses aim to pinpoint the sources of ligands and receptors involved in the process.
By elucidating the molecular players and the tempo of this specification process, we aim to develop a feasible protocol for the efficient generation of thyroid organoids without genetic manipulation of the cells, making them suitable for future cell therapy applications.
Benefits
As a PhD student in our team, you will benefit from expert mentorship, cutting-edge imaging and cell biology facilities and collaborate closely with experts in organoid technology. You will engage in a multidisciplinary project encompassing organoids, gene editing, imaging and transcriptomics, addressing significant questions in the fields of thyroid and developmental biology.
Key tasks and responsibilities:
- Implement new models of organoids.
- Cellular and molecular characterization of those organoids
- Conduct single-cell RNA sequencing, single-seq ATAC sequencing and transcriptomic and genomic analysis.
Qualifications:
- Master in Biology, medical biology, Bioengineering or a related field.
This project is ideal for an ambitious and enthusiastic candidate who thrives in a dynamic environment. Proficiency in scientific English—speaking, reading, and writing—is essential.
To complete your application (see documents on our website, https://iribhmphd.ulb.be), kindly provide a cover letter detailing your research interests and career aspirations, along with an updated CV and the contact details of two or three references.
To apply: https://iribhmphd.ulb.be
Contact: Sabine Costagliola sabine.costagliola@ulb.be
——————————————————————————————————————————————
PhD position on the effect of air pollution on developing and mature human lung organoids
This PhD position is supervised by Mírian Romitti, a new young PI at IRIBHM and it is based in the IRIBHM-Jacques Dumont Institute (Faculté de Medicine) at the Université Libre de Bruxelles (ULB). The hosting institute/university is international, inclusive, and well-known for its significant contributions to basic and translational fields. The proposed topic is part of a larger project based on using stem cell-derived organoids to study human lung development and to model pulmonary diseases. The student will be part of a young and energetic team and work in close collaboration with other master’s and PhD students, as well as with a group of collaborators holding great expertise in bioengineering, bioinformatics, immunology, toxicology, and medical doctors.
About the project
It is well established that exposure to particle pollution is a significant threat to health at distinct stages of human life, resulting in premature birth, causing/worsening heart and lung diseases, and consequently shortening lives. In the case of the lungs, exposure to pollution impacts its growth and function, both prenatally and postnatally. In young kids, ambient pollutants increase the risk of respiratory infections, developing or worsening asthma, and chronic diseases. Recent data has demonstrated that black carbon (BC) particles can be found in fetal lungs during the first and second trimesters of pregnancy. Despite the shreds of evidence of particle accumulation in fetal and adult lungs little is known of how it can impact development and physiology.
Studying mechanistic aspects of human diseases has been challenging due to the species-specificities and the difficulties in accessing human samples. However, organoid technology emerged as a great tool filling the gap in human research. Our lab recently generated a novel human lung organoids model derived from human stem cells (hESCs) that derives the full organ. This new system allows live tracking of the lung developmental stages and generates both the epithelial (airways and alveoli) and the stromal compartment of the organ.
Major goal: Dissect how ambient pollutants (BCs) can affect lung development (“fetal” stages) and function (“adult”) by evaluating their deposition among the lung cells and how it can affect the cell state at transcriptional, morphological, and functional levels, using human lung organoids as a research model.
Publications:
Lasolle, A. Schiavo, A. Tourneur, P. Gillotay, B. de F. da Fonseca, L. Ceolin, O. Monestier, B. Aganahi, L. Chomette, M. M. L. Kizys, L. Haenebalcke, T. Pieters, S. Goossens, J. Haigh, V. Detours, A. L. S. Maia, S. Costagliola, and M. Romitti, Dual targeting of MAPK and PI3K pathways unlocks redifferentiation of Braf-mutated thyroid cancer organoids. Oncogene 1–16 (2023) doi:10.1038/s41388-023-02889-y.
Mírian Romitti, Barbara de Faria da Fonseca, Gilles Doumont, Pierre Gillotay, Adrien Tourneur, Sema Elif Eski, Gaetan Van Simaeys, Laura Chomette, Helene Lasolle, Olivier Monestier, Dominika Figini Kasprzyk, Vincent Detours, Sumeet Pal Singh, Serge Goldman, Samuel Refetoff, Sabine Costagliola, « Transplantable human thyroid organoids generated from embryonic stem cells to rescue hypothyroidism » Nat Commun 13, 7057 (2022). https://doi.org/10.1038/s41467-022-34776-7
Main techniques to be used (experience not mandatory)
- hESCs culture.
- Organoid cell culture using static and microfluidics conditions (interaction with bioengineers).
- Co-culture with immune cells.
- Single Cell Transcriptomics (including spatial scRNAseq) and Chromatin Remodeling assessments (ATACseq) (interaction and training with bioinformaticians).
- Immunofluorescence, Electron Microscopy, MERFISH and Flow cytometry.
- Live imaging and high-resolution microscopy.
- Black carbon detection (femtosecond pulsed illumination in collaboration with the University of Hasselt).
What we expect from you
- Motivated candidate holding a Master’s degree (or graduating) in degree in medicine, biomedical sciences, pharmaceutical sciences, biology, bioengineering, biochemical sciences, or a related field.
- Ability to work in a team of young-friendly scientists, to be flexible, and to incorporate into a fast-paced environment.
- The capacity to work independently while keeping a strong team spirit.
- Strong self-motivation, organizational skills, and ability to lead and develop scientific projects.
- Good English language skills (speaking, reading, and writing) are also required.
What you will find
- The PhD student will be supported by a Thesis Advisory Committee, participate in scientific and professional skills courses, and attend international conferences.
- Weekly, the student will participate in a lab meeting with other teams working on organoids.
- The Institute provides an interactive environment and the student will have the opportunity to interact with students/researchers working on many distinct fields.
- Since the project involves collaborations with teams inside and outside ULB, interaction with those teams will be part of the PhD training and the student will have the possibility to learn new technologies/techniques in those other laboratories.
To apply: https://iribhmphd.ulb.be
Contact: Mírian Romitti – mirian.romitti@ulb.be
Postdoc Positions
Postdoc position in organoids research for modeling rare diseases
The Costagliola lab, at IRIBHM, ULB, Brussels, (https://costalab.ulb.ac.be/ ; https://iribhm.org/) is seeking a creative, motivated and highly independent postdoctoral fellow with a good expertise in Human stem cell-derived organoids. The project aims to uncover the molecular pathways responsible for resistance to Thyrotropin, a rare human disorder initiated by a newly identified genetic variation unique to primates.
This position is fully funded for 3 years. Salary commensurate with experience.
Project Background
The thyroid gland’s primary role is to capture iodide and synthesize Thyroid hormones (TH), crucial for normal growth and metabolism, which exert effects on nearly all tissues. Resistance to Thyroid-Stimulating Hormone (RTSH) denotes a syndrome where the thyroid displays variable hypersensitivity to biologically active TSH, a hormone from the pituitary gland. This condition is characterized by elevated serum TSH levels, a normal or underdeveloped thyroid, and varying serum TH levels, dependent on the degree of TSH insensitivity. RTSH is categorized as fully compensated, partially compensated, or uncompensated based on TH levels, with the latter indicating hypothyroidism due to low TH production. While loss-of-function mutations in the TSH receptor are a common cause of RTSH, an identical phenotype has been observed without such mutations, suggesting alternative genetic factors. In some patients, dominantly inherited RTSH has been linked to mutations in a Short Tandem Repeat (STR) in several families with RTSH (in press). Our proposed project aims to utilize human thyroid organoids as a model for RTSH. Based on our expertise in generating functional human thyroid organoids from pluripotent stem cells (Antonica et al, Nature 2012; Romitti et al, Nat. Commun, 2022) and genome editing of stem cell lines, we will conduct functional assays, transcriptome and chromatin accessibility analyses in both wild-type and mutated organoids. This approach will enable us to elucidate the underlying molecular mechanisms contributing to RTSH. Interestingly, this STR is exclusive to primates, prompting an additional aspect of the project to explore its role in evolutionary endocrinology.
Postdoc benefits
As a postdoctoral researcher in our team, you will benefit from cutting-edge imaging and cell biology facilities and collaborate closely with experts in organoid technology. You will engage in a multidisciplinary project encompassing organoids, gene editing, imaging and transcriptomics, addressing significant questions in the fields of thyroid biology and evolutionary endocrinology. Candidates should demonstrate a track record of research productivity through publications.
Key tasks and responsibilities:
- Implement new models of Thyroid organoids.
- Generate genetically modified/edited human stem cells lines.
- Conduct single-cell RNA sequencing and transcriptomic analysis.
Qualifications in several of the following areas:
- PhD in Biology, Bioengineering or a related field.
- Proficiency in Human and/or mouse stem cell-derived organoids
- Experience in CRISPR-based genome editing.
- Experience in Confocal microscopy and image processing
- Experience in Bioinformatic for transcriptome analysis
- Developmental biology
This project is ideal for an ambitious and enthusiastic candidate who thrives in a dynamic environment. Proficiency in scientific English—speaking, reading, and writing—is essential.
To complete your application, kindly provide a cover letter detailing your research interests and career aspirations, along with an updated CV and the contact details of three references.
Contact: Sabine Costagliola sabine.costagliola@ulb.be
Lab technician Positions
Technicien.ne de laboratoire pour le développement d’organoïdes cellulaires pour modéliser des pathologies humaines (IRIBHM-ULB, Faculté de médecine)
Description de l’offre Le laboratoire de développement d’organoïdes humains à partir de cellules souches dirigé par le Professeur Sabine Costagliola est un laboratoire de recherche de l’ Institut de Recherche en Biologie humaine et Moléculaire (IRIBHM) de l’Université libre de Bruxelles (ULB) situé sur le campus Erasme à Anderlecht. Ce laboratoire développe des organoïdes humains (Mini-organes in Vitro) pour modéliser le développement et les pathologies thyroïdiennes et pulmonaires. Le/la technicien.ne travaillera dans un environnement international et participera à des projets de recherche de pointe. https://iribhm.org/
Missions: Pratiquer les techniques usuelles de biologie moléculaire (extraction d’ARN, qPCR..) Préparer des cultures cellulaires de cellules souches et d’organoïdes Effectuer des coupes au cryostat et coupes en paraffine Effectuer de l’immunohistochimie Acquisition d’images par microscopie à fluorescence
Profil : Être titulaire d’un bachelier ou d’un master en sciences ou en biologie médicale reconnu par la FWB Les personnes possédant un diplôme n’émanant pas de la Fédération Wallonie Bruxelles ni de la Communauté flamande devront en obtenir une équivalence par la FWB : (http://www.equivalences.cfwb.be/index.php?id=486)
Connaissances et compétences requises: Bonne connaissance de l’anglais (B2) Techniques de biologie moléculaire et de culture cellulaire (requises)
Qualités: Motivation et dynamisme Capacité rapide d’intégration et d’apprentissage Esprit d’équipe Proactivité Curiosité
Nous offrons: Un cadre de travail agréable et dynamique au sein d’une institution avec des valeurs sociétales fortes Une formation permanente pour enrichir son CV Une échelle barémique conforme aux barèmes de la FWB qui tient compte de l’expérience acquise (sous conditions) Des avantages extra légaux (régime de congés attrayant, possibilité d’affiliation à une assurance hospitalisation, pension complémentaire, intervention dans les frais de déplacement…)
Complément d’information CDD d’un an à temps plein renouvelable
Personnes de contact Professeur Sabine Costagliola Sabine.costagliola@ulb.be Véronique Janssens, Lab Manager janssens.veronique@ulb.be
News

H2020 Project
New European H2020 research project
The influence of endocrine disruptors on the male and female thyroid gland. The new European collaborative project SCREENED aims to develop three-dimensional (3D) cell-based in vitro tests to better characterize the effects of endocrine disruptors on thyroid functions. This method will overcome the limitations of existing tests, being more sensitive, at low doses of exposure to chemicals, and enabling the prediction of their toxicity on human health in a sex-specific manner. Sabine Costagliola Lab at IRIBHM is the Belgian partner of this ambitious and innovative project.

This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 825745.
Contact:
Sabine Costagliola
ULB – IRIBHM, Faculty of Medicine
scostag@ulb.ac.be